Foot & Ankle Stents
GraMedica®

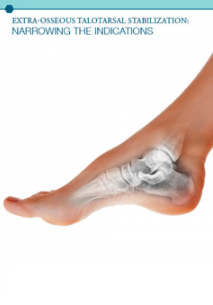

GraMedica® was founded in 2002 by Dr. Michael E Graham, a successful, Michigan-based doctor of Podiatric Medicine. In his practice, Dr. Graham specialized in the correction of foot and ankle instability, a root cause of many secondary conditions throughout the body. It had long been known that the underlying cause of many foot and ankle problems is excessive hindfoot motion. Frustrated by the limitations of available treatment options on the market, Dr. Graham was determined to improve patient outcomes by providing a revolutionary solution to answer this challenge. Dr. Graham invented HyProCure®, an Extra-Osseous TaloTarsal Stabilization (EOTTS) Device.
This pioneering device is placed deep into the canalis portion of the sinus tarsi reestablishing the normal pivot over which the talus (ankle bone) glides, thereby properly re-aligning the foot and ankle bones and restoring normal function. HyProCure® received FDA approval on September 16,= 2004. The first HyProCure® placement took place on September 22, 2004. As more and more doctors and patients gained awareness of both the condition and treatment, the company experienced rapid growth in July 2011. GraMedica® received FDA clearance for its second implantable device, osteo- WEDGE™, a revolutionary treatment option for moderate to severe bunion correction.
